Abstract
Background
Multiple myeloma (MM) is the second-most common hematological cancer worldwide. Patients with MM experience substantial symptom burden and reduction in health-related quality of life (HRQoL) compared with the general population; therefore, maintaining good quality of life is a key goal of therapy. Our systematic review aimed to better understand the impact of treatment response and disease progression on HRQoL in patients with MM.
Methods
A literature search in accordance with PRISMA guidelines was conducted on January 14, 2022, to identify studies investigating HRQoL in adults with MM. We searched multiple databases (Embase, MEDLINE and Cochrane), and conducted a grey literature search by reviewing bibliographies of relevant review articles. Titles and abstracts were independently screened by two reviewers for inclusion based on pre-defined Population, Intervention, Comparator, Outcomes and Study Design criteria. Records flagged for inclusion had full texts subsequently screened using the same method. A third round of screening was conducted to identify studies that discussed the relationship of HRQoL to disease progression, line of therapy, or response. Quality assessment was conducted on utility studies using the National Institute for Health and Care Excellence Quality Assessment Checklist for Health State Utility Values.
Results
In total, 2198 unique records were identified through database searching and 872 records were identified through a grey literature search. After screening these records, we identified 44 records (representing 41 studies) for formal inclusion in the qualitative analysis. 30 records reported data relating HRQoL to disease progression, 5 reported data relating HRQoL to line of therapy, and 19 reported data relating HRQoL to response. Records that reported data from different areas of interest were counted in multiple respective categories.
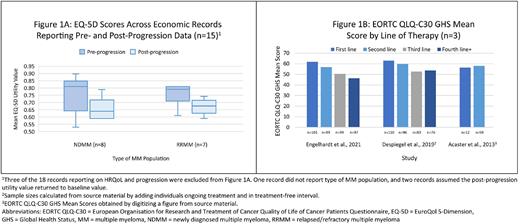
Of the 30 progression records, 18 were economic studies reporting utility values. Both relapsed/refractory MM (RRMM) and newly diagnosed MM (NDMM) patients showed worsening utility values with disease progression. The median utility value pre-progression for NDMM patients was 0.81. Post-progression, the median fell to 0.64. For RRMM patients, these values were 0.79 and 0.68, respectively (Figure 1A). Four records reported utility values from non-economic studies. Patients across these studies experienced an improvement from baseline utility during treatment, followed by a decrease in utility at the time of disease progression. In 11 records, non-utility HRQoL data was reported. Six of these records were based on longitudinal studies that showed patients' HRQoL worsened at the time of progressive disease in most domains. Five cross-sectional records showed patients with progressive disease had decreased HRQoL compared with patients in other disease states.
Of the five line of therapy records, four unique studies were represented. Three studies reported decreasing HRQoL with increasing lines of therapy; the fourth did not find a consistent association. Non-utility HRQoL data by line are shown in Figure 1B.
Of the 19 response records, 14 looked directly at the relationship between HRQoL and varying levels of response and found increasing depth of response was associated with improved patient HRQoL. A greater depth of response and achieving minimal residual disease-negative status were associated with a longer time to worsening of HRQoL scores in three records.
Conclusion
Our systematic review suggests improved HRQoL is related to desirable treatment outcomes such as delayed progression, fewer lines of therapy, and achieving the deepest possible clinical response. These findings suggest disease response and progression are valuable patient-relevant endpoints. However, the heterogeneity in study design and limited number of studies available relating HRQoL to disease progression, line of therapy, and/or response indicate further directed research is needed in this area to best improve patient outcomes. HRQoL measures should be collected systematically in an ongoing manner in future clinical trials and observational studies in patients with MM.
Disclosures
Fonseca:Adaptive Biotechnologies: Divested equity in a private or publicly-traded company in the past 24 months; Genentech, Pfizer, Sanofi: Honoraria, Research Funding; FIH prognostication in myeloma: Patents & Royalties; Adaptive Biotechnologies, Caris Life Sciences, OncoMyx and OncoTracker: Other: Scientific Advisory Board; AbbVie, Amgen, Bayer, BMS/Celgene, GSK, H3 Therapeutics, Janssen, Juno, Karyopharm, Kite, Merck, Novartis, Oncopeptides, OncoTracker, Pfizer, Pharmacyclics, Regeneron, Sanofi, Takeda: Consultancy; Adaptive Biotechnologies, Caris Life Sciences, Oncomyx and OncoTracker: Membership on an entity's Board of Directors or advisory committees; Amgen, BMS, Celgene, Takeda, Bayer, Janssen, Novartis, Pharmacyclics, Sanofi, Merck, Juno, Kite, Aduro, OncoTracker, GSK, AbbVie, Pfizer, Karyopharm.: Consultancy. Tran:EVERSANA, which is a hired consultancy to Janssen and other pharmaceutical, medical device and biotechnology companies: Current Employment. Rosta:EVERSANA, which is a hired consultancy to Janssen and other pharmaceutical, medical device and biotechnology companies: Current Employment. Laidlaw:EVERSANA, which is a hired consultancy to Janssen and other pharmaceutical, medical device and biotechnology companies: Current Employment. Rai:EVERSANA, which is a hired consultancy to Janssen and other pharmaceutical, medical device and biotechnology companies: Current Employment. Duran:Janssen Research and Development: Current Employment. Ammann:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal